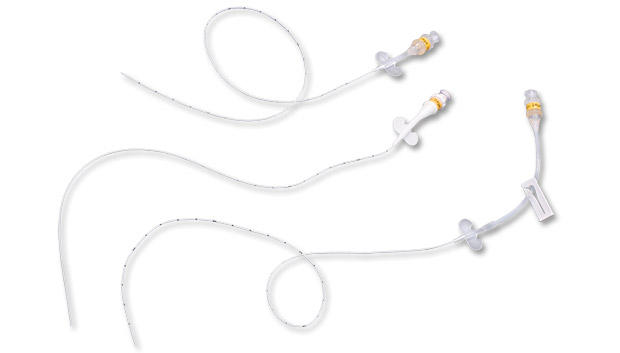
PICC-Nate® is used for central venous catheter indwelling longevity while minimizing neonatal trauma. Multiple special design features have been incorporated at clinician request including developmentally friendly materials, low profile and extended hub configurations and intracorporeal positioning aids.
PRODUCT DESCRIPTION
PICC-Nate® is a peripherally inserted central catheter specially designed to avoid irritating the baby’s delicate tissues.
FEATURES
INDICATIONS
PICC-Nate® is designed for use when long term central venous catheterization is prescribed.
CONTRAINDICATIONS
• Lack of qualified healthcare practitioner for catheter insertion and placement.
• Known or suspected catheter-related infection.
• Patient's vasculature insufficient to accommodate size of catheter.
• Previous trauma of potential insertion site.
• Previous episodes of venous thrombosis, or previous vascular surgical procedure at the potential insertion site.
• Inability to properly stabilize catheter at insertion site.
• Previously opened or damaged catheter package, or past expiration date.
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.
© Copyright 2024 Utah Medical Products, Inc. All rights reserved.